Germination, Morphological and Physiological Evaluation of Seedlings Pretreated with Colchicine in Soybean (Glycine max L)
DOI:
https://doi.org/10.48048/wjst.2021.9489Keywords:
Colchicine, Germination, Growth, Morpho-physiological parameters, SoybeanAbstract
Wider genetic diversity has the potential to improve crop productivity of soybean, especially under environmental stress conditions. The pre-treatment of soybean seeds with antimitotic agents to establish improved genetic pool may also contribute to the enhancement of germination, seedling development, morpho-physiological growth and yield. In this study, 2 soybean genotypes viz. TGx1835-10E and Dundee were imbibed in solutions containing different amounts of colchicine (0.0, 0.1, 0.5 and 1 %) to evaluate the variations in germination, morphometric and physiological parameters. The seeds were imbibed for the period of 12 and 24 h before sowing for germination in plastic pots containing moistened sterile vermiculite. The variance components expressed as means, and mean percentage of total variations showed that colchicine concentration and imbibitional duration were the most important sources of variation for all traits, followed by the genotypes. Significant responses were detected for various germination parameters, seedling morphology and physiological contents such as; chlorophyll content, total phenolics, flavonoids as well as total protein and DNA content in the 2 genotypes used.
HIGHLIGHTS
- Mutagenic pre-treatment of seeds via imbibition
- Germination and seedling growths of colchicine preconditioned seeds
- Chloroplastidic pigment analysis of pre-treated seedlings
- Physiological valuation of primary and secondary metabolites in grown seedlings
- Role of colchicine on germination, seedling development and growth of soybean plantlets
GRAPHICAL ABSTRACT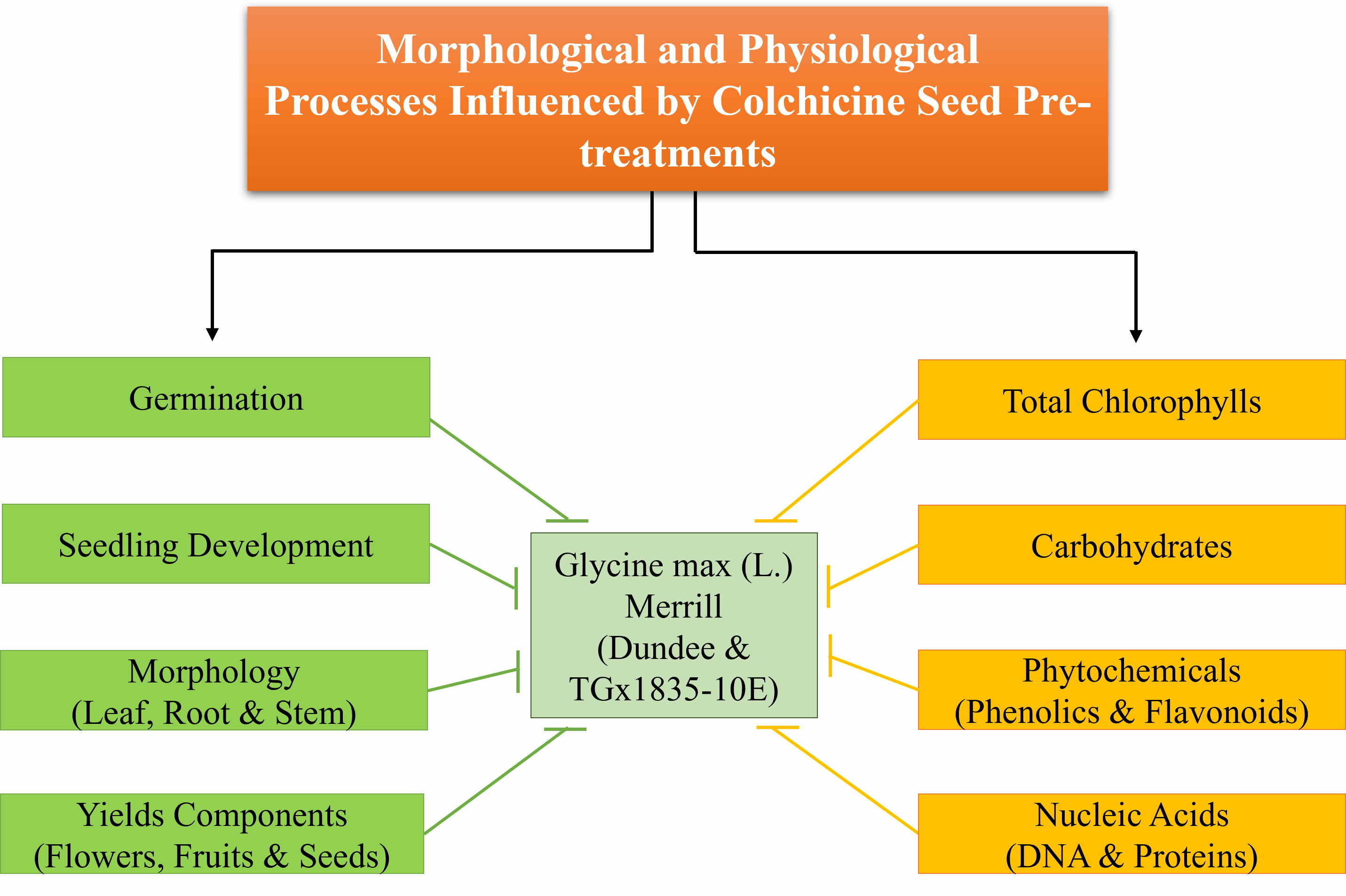
Downloads
Metrics
References
WH Jeong, K Harada, T Yamada, J Abe and K Kitamura. Establishment of new method for analysis of starch contents and varietal differences in soybean seeds. Breed Sci. 2010; 90, 160-3.
A Manzoor, T Ahmad, MA Bashir, IA Hafiz and C Silvestric. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants. 2019; 8, 194.
E Essel, IK Asante and E Laing. Effect of colchicine treatment on seed germination, plant growth and yield traits of cowpea (Vigna unguiculata (L.) Walp). Canadian J. Pure. Appl. Sci. 2015; 9, 3573-6.
P Mangena, PW Mokwala and RV Nikolova. In vitro multiple shoot induction in soybean. Int. J. Agric. Biol. 2015; 17, 838-42.
RF Boyer. Modern Experimental Biochemistry. 2nd Edition. Benjamin Cummings, San-Francisco, USA, 1993, p. 379-85.
AM Torres, T Mau-Lastovicka and R Rezaainyan. Total phenolics and high-performance liquid chromatography of phenolics in avocado. J. Agric. Food Chem. 1987; 35, 921-5.
M Mingozzi, M Madeo and G Speranza. Effect of nitrogen starvation on the phenolic metabolism and antioxidant properties of yarrow. Food Chem. 2009; 114, 204-11.
D Marinova, F Ribarova and M Atanassova. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Metall. 2005; 40, 255-60.
J Zhishen, T Mengcheng and W Jianming. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64, 555-9.
T Masuko, A Minami, N Iwasali, T Majima, S Nishimura and YC Lee. Carbohydrates analysis by phenol-sulfuric acid method in microplate format. Ana Biochem. 2005; 339, 69-27.
CF Barbas, DR Burton, JK Scott and GJ Silverman. Quantitation of DNA and RNA. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA, 2007.
OG Bhusnure, VS Kuthar, SB Gholve, PP Jadhav, SG Zingade and PS Giram. Spectrophotometric method for quantitative estimation of DNA isolated from various parts of Catharanthus roseus Linn. J. Pharm. Res. 2017; 11, 625-8.
ISTA-International Seed Testing Association. International rules for seed testing. Seed Sci. Tech. 1993; 29, 142-68.
FJ Czabator. Germination value: An index combining speed and completeness of pine seed germination. For Sci. 1962; 8, 386-96.
MA Kader. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. Roy. Soc. New South Wales. 2005; 138, 65-75.
KC Gairola, AR Nautiyal and AK Dwivedi. Effect of temperature and germination media on seed germination of Jatropha curcas Linn. Adv. Bio-Res. 2011; 2, 66-71.
AKMA Islam, N Anuar and Z Yaakob. Effect of genotypes and pre-sowing treatment on seed germination behaviour of Jatropha. Asian J. Plant Sci. 2009; 8, 433-9.
M Nikolova, M Petrova, E Zayoza, A Vitkova and L Evstatieva. Comparative study of in vitro, ex vitro and in vivo grown plants of Arnica montana-polyphenols and free radical scavenging activity. Acta Bot. Croat. 2013; 72, 13-22.
A Kafeel, ZI Khan, ZA Shah, M Irahim, I Mustafa and EE Valeem. Evaluation of available sugars in plant species indigenous to soone valley (Punjab) Pakistan. Pak. J. Bot. 2008; 40, 1877-83.
JF Fannie and VJ Stadan. Gloriossa superba L. (Flame lily): micropropagation and in vitro production of colchicine. Medic. Arom. Plants. 1994; 6, 146-66.
D Dhakhanamoorthy, R Selvaraj and A Chidambaram. Physicla and chemical mutagenics in Jatropha curcas L. to induce variability in seed germination, growth and yield traits. Rom. J. Plant Biol. 2010; 55, 113-25.
S Pande and M Khetmalas. Biological effect of sodium azide and colchicine on seed germination and callus induction in Stevia rebaudiana. Asian J. Exp. Biol. Sci. 2012; 3, 93-8.
W Widoretno. In vitro induction and characterization of tetraploid patchouli (Pogostemon cablin Benth.) plant. Plant Cell Tiss. Organ Cult. 2016; 125, 261-7.
S Khalili, M Niazian, A Mustafa and M Norouzi. In vitro chromosome doubling of African daisy, Gerbera jamesonii Bolus cv. Mini Red. The Nucleus. 2020; 63, 59-65.
OU Udensi and V Ontui. Determination by flow cytometry polyploidy inducing-capacity of colchicine in Cajanus cajan (L.) Mill sp. Parkistan J. Biol. Sci. 2013; 16, 630-5.
S Amiri, SK Kazemitabaar, G Ranjbar and M Azadbakht. The effect of trifluralin and colchicine treatments on morphological characteristics of jimson weed (Datura stramonium L.). Trakia J. Sci. 2010; 8, 47-61.
S Chandra, S Khan, B Avula, H Lata, MH Yang, MA Elsohly and IA Khan. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evi-Based Compl. Alter. Med. 2014; 253875, 1-9.
EA Mogotlane, PW Mokwala and P Mangena. Comparative analysis of the chemical compositions of indigenous watermelon (Citrullus lanatus) seeds from two districts in Limpopo Province, South Africa. Afri J. Biotech. 2018; 17, 1001-6.
RH Nieman and LL Poulsen. Spectrophotometric estimation of nucleic acid of plant leaves. Plant Physiol. 1963; 38, 31-5.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.