The Effect of Carboxymethyl Glucomannan Concentration on the Properties of Glucomannan-Chitosan Hydrogel for Lactobacillus acidophilus FNCC 0051 Encapsulation
DOI:
https://doi.org/10.48048/wjst.2021.22787Keywords:
Chitosan, Encapsulation, Glucomannan, Hydrogel, Probiotic, PropertiesAbstract
The effect of porang (Amorphophallus oncophyllus) glucomannan concentration on the properties of glucomannan-chitosan hydrogel was investigated for Lactobacillus acidophilus FNCC 0051 encapsulation. The spherical shape with a continuous surface of the particle was self-assembly formed. The increase of glucomannan concentration from 0.3 to 0.9 % smoothly increased their small particle size from 1.08 ± 0.02 µm to 2.12 ± 0.00 µm and no significant change on the positive zeta potential values. The polydispersity indexes with the value between 0.4 to 0.5 were categorized as uniform particles. However, these values were higher compared to other studies which used konjac glucomannan-chitosan as the hydrogel materials. The encapsulation study with Lactobacillus acidophilus FNCC 0051 showed that the highest value was achieved when the same ratio of glucomannan and chitosan was applied (0.5 %). The viability study proved the perfect protection of hydrogel during 56 days of cold storage and pasteurization treatment with the cell viabilities of 100 % and 58.13 ± 18.5 %, respectively.
HIGHLIGHTS
- Glucomannan concentration influenced particle size and encapsulation efficiency of hydrogel
- Hydrogel was potential as acidophilus carrier in the gut due to its pH sensitivity
- Hydrogel had continuous surface to provide a stronger physical barrier for the acidophilus against harsh environment
- Hydrogel proved well protection of acidophilus during pasteurization and cold storage
GRAPHICAL ABSTRACT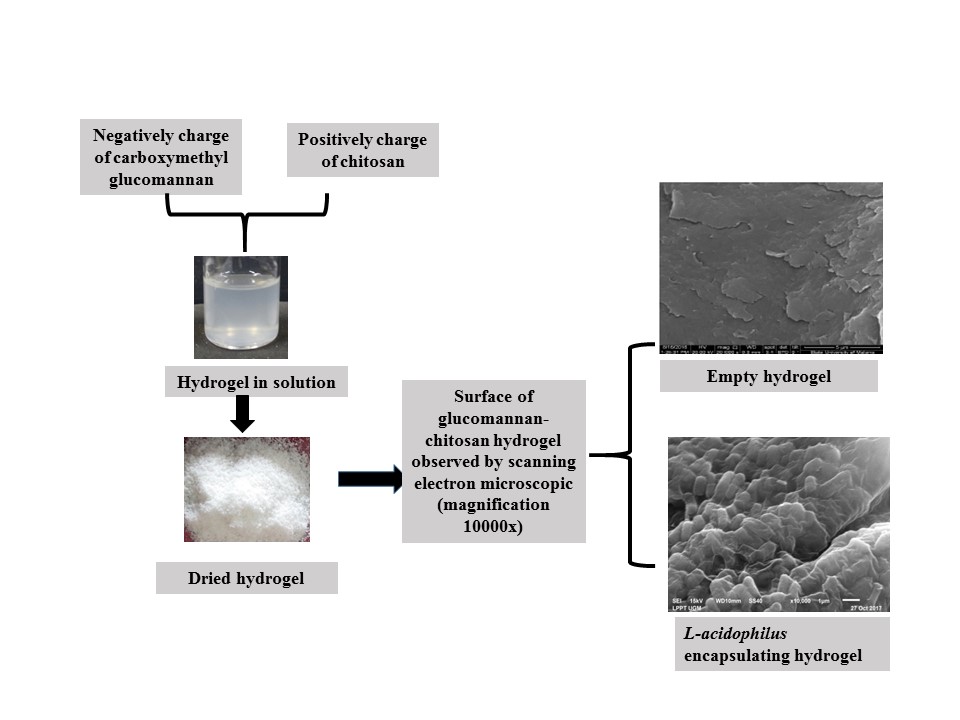
Downloads
Metrics
References
Food and Agriculture Organization. Probiotics in food: Health and nutritional properties and guidelines for evaluation. Food and Agriculture Organization of the United Nations, Rome, 2006.
YK Lee and S Salminen. Handbook of probiotics and prebiotics. John Wiley and Sons, New Jersey, 2008.
NJ Zuidam and VA Nedovic. Encapsulation technologies for active food ingredients and food processing. Springer, New York, 2010.
AJ Priya, SP Vijayalakshmi and AM Raichur. Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. J. Agr. Food Chem. 2011; 59, 11838-45.
SA Rather, R Akhter, FA Masoodi, A Gani and SM Wani. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei. NCDC297. LWT Food Sci. Tech. 2017; 83, 50-8.
M Halim, NAM Mustafa, M Othman, H Wasoh, MR Kapri and AB Ariff. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT Food Sci. Tech. 2017; 81, 210-6.
LA Bosnea, T Moschakis and CG Biliaderis. Complex coacervation as a novel microencapsulation technique to improve viability of probiotics under different stresses. Food Bioproc. Tech. 2014; 7, 2767-81.
M Xu, F Gagné-Bourque, MJ Dumont and S Jabaji. Encapsulation of Lactobacillus casei ATCC 393 cells and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J. Food Eng. 2016; 168, 52-9.
J Yang, S Han, H Zheng, H Dong and J Liu. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015; 123, 53-66.
Y Li. 2011, Smart microgels for controlled uptake and release. Ph.D. Dissertation. Wageningen University, Wageningen, The Netherlands.
E Harmayani. Metode Ekstraksi Glukomanan dari Tepung Umbi Porang (Amorphophallus oncophyllus). P00201601183, 2016.
A Yanuriati, DW Marseno, Rochmadi and E Harmayani. Characteristics of glucomannan isolated from fresh tuber of Porang (Amorphophallus muelleri Blume). Carbohydr. Polym. 2017; 156, 56-63.
E Harmayani, V Aprilia and Y Marsono. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr. Polym. 2014; 112, 475-9.
J Fan, K Wang, M Liu and Z He. In vitro evaluations of konjac glucomannan and xanthan gum mixture as the sustained release material of matrix tablet. Carbohydr. Polym. 2008; 73, 241-7.
T Ye, L Wang, W Xu, J Liu, Y Wang, K Zhu, S Wang, B Lin and C Wang. An approach for prominent enhancement of the quality of konjac flour: Dimethyl sulfoxide as medium. Carbohydr. Polym. 2014; 99, 173-9.
H Yu and C Xiao. Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr. Polym. 2008; 72, 479-89.
J Du, J Dai, J Liu and T Dankovich. Novel pH-sensitive polyelectrolyte carboxymethyl Konjac glucomannan-chitosan beads as drug carriers. React. Funct. Polym. 2006; 66, 1055-61.
L Wang, M Xiao, S Dai, J Song, X Ni, Y Fang, H Corke and F Jiang. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014; 101, 136-45.
S Korkiatithaweechai, P Umsarika, N Praphairaksit and N Muangsin. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011; 9, 1649-63.
NT Annan, AD Borza and LT Hansen. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008; 41, 184-93.
GK Gbassi and T Vandamme. Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics 2012; 4, 149-63.
E Valero-Cases and MJ Frutos. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT Food Sci. Tech. 2015; 64, 824-8.
R Vidhyalakshmi, R Bhakyaraj and R Subhasree. Encapsulation “the future of probiotics”: A review. Biol. Res. 2009; 3, 96-103.
V Aprilia, A Murdiati, P Hastuti and E Harmayani. Encapsulation of Lactobacillus acidophilus FNCC 0051 in hydrogel using a complex coacervation of glucomannan and chitosan. Res. J. Microbiol. 2017; 12, 236-42.
P Chitprasert, P Sudsai and A Rodklongtan. Aluminum carboxymethyl cellulose - rice bran microcapsules: Enhancing survival of Lactobacillus reuteri KUB-AC5. Carbohydr. Polym. 2012; 90, 78-86.
S Sathyabama, M Ranjith, P Bruntha, R Vijayabharathi and V Brindha priyadharisini. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Tech. 2014; 57, 419-25.
PK Okuro, M Thomazini, JCC Balieiro, RDCO Liberal, CS Fávaro-trindade. Co-encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013; 53, 96-103.
D Charalampopoulos and RA Rastall. Prebiotics and probiotics science and technology. Springer, New York, 2009.
JM Lakkis. Encapsulation and controlled release technologies in food systems. Blackwell Publishing, Iowa, 2007.
J Du, R Sun, S Zhang, T Govender, LF Zhang, CD Xiong and YX Peng. Novel polyelectrolyte carboxymethyl konjac glucomannan-chitosan nanoparticles for drug delivery. Macromol. Rapid Comm. 2004; 25, 954-8.
J Du, R Sun, S Zhang, LF Zhang, CD Xiong and YX Peng. Novel polyelectrolyte carboxymethyl konjac glucomannan-chitosan nanoparticles for drug delivery. I. Physicochemical characterization of the carboxymethyl konjac glucomannan-chitosan nanoparticles. Biopolymers 2005; 78, 1-8.
HM Shewan and JR Stokes. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013; 119, 781-92.
AKAS Brun-graeppi, C Richard, M Bessodes, D Scherman and OW Merten. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Control. Release 2011; 149, 209-24.
PD Gaudio, P Colombo, G Colombo, P Russo and F Sonvico. Mechanisms of formation and disintegration of alginate beads obtained by prilling. Int. J. Pharm. 2005; 302, 1-9.
R Wang, B Xia, B Li, S Peng, L Ding and S Zhang. Semi-permeable nanocapsules of konjac glucomannan – chitosan for enzyme immobilization. Int. J. Pharm. 2008; 364, 102-7.
T Egan, D O’Riordan, M O’Sullivan and JC Jacquier. Cold-set whey protein microgels as pH modulated immobilisation matrices for charged bioactives. Food Chem. 2014; 156, 197-203.
H Yu, J Lu and C Xiao. Preparation and properties of novel hydrogels from oxidized konjac glucomannan cross-linked chitosan for in vitro drug delivery. Macromol. Biosci. 2007; 7, 1100-11.
C Alvarez-Lorenzo, B Blanco-Fernandez, AM Puga and A Concheiro. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013; 65, 1148-71.
Z Jiang, B Han, H Li, Y Yang and W Liu. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015; 129, 1-8.
AM Mortazavian, MR Ehsani, SM Mousavi, K Rezaei, S Sohrabvandi and JA Reinheimer. Effect of refrigerated storage temperature on the viability of probiotic micro-organisms in yogurt. Int. J. Dairy Tech. 2007; 60, 123-7.
FCA Buriti, TR Komatsu and SMI Saad. Activity of passion fruit (Passiflora edulis) and guava (Psidium guajava) pulps on Lactobacillus acidophilus in refrigerated mousses. Braz. J. Microbiol. 2007; 38, 315-7.
S Rathore, PM Desai, CV Liew, LW Chan and PWS Heng. Microencapsulation of microbial cells. J. Food Eng. 2013; 116, 369-81.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.













