Factors Contributing to the Clay Dispersion and Aggregate Stability of Thai Oxisols
Keywords:
Soil moisture regime, soil properties, clay dispersion, aggregate stability, Thai OxisolsAbstract
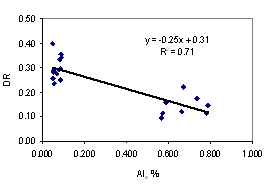
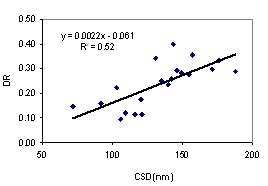
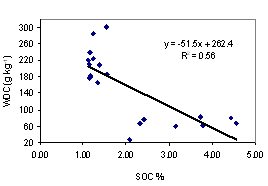
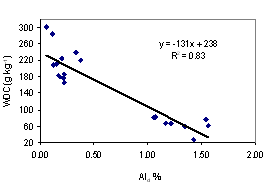
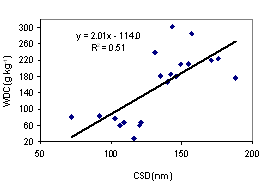
Aggregate stability tends to give a better availability of soil water. Clay dispersion makes the soil dense, difficult for root penetration. The better understanding of these two soil properties is necessary to evaluate the suitability of soils for plant production. We collected soil samples from 10 sites from the southeast coast and northeast plateau of Thailand, at upper (0 - 5 cm depth) and lower (5 - 20 cm depth) levels for under udic and ustic Oxisols soil samples. The objective of this study was to investigate the contribution of major aggregate binding agents such as soil organic carbon (SOC), Fe and Al oxides, clay minerals and kaolin crystal size to the stability of soil aggregates. Soils were acidic (pH < 6.5), low to medium cation exchange capacity (3.34 - 14.40 cmol kg-1), SOC ranged from 2.08 - 4.56 % for udic and 1.11 - 1.56 % for ustic soils. The udic soils showed higher contents of crystalline, non-crystalline, and organic forms of Fe and Al than those for ustic soils. The mean coherently scatterings domain (CSD) values for kaolin crystal size were 115 and 152 nm for udic and ustic soils, respectively. The mean water dispersible clay (WDC), water dispersible silt (WDSi), and dispersion ratio (DR) were 92, 43 g kg-1, and 0.17 for udic soils, and 218, 45 g kg-1, and 0.30 for ustic soils, respectively. Mean weight diameters (MWD) were 1.01 and 0.62 mm for udic and ustic soils, respectively. The clay flocculation index (CFI) and aggregated silt and clay (ASC) were 0.83 and 650 g kg-1 for udic soils and 0.66 and 619 g kg-1 for ustic soils. The SOC, non-crystalline, organic form of Fe and Al oxides, and crystalline Al oxide showed positive correlation to MWD and CFI. A negative correlation between crystal size of kaolin and aggregate stability was found.
Graphical abstract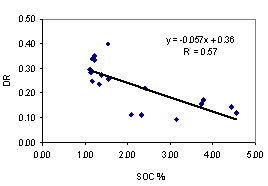
Downloads
Metrics
References
JM Oades. Soil organic matter and structure stability, mechanism and implication for measurement. Plant and Soil 1984; 76, 319-37.
JM Lima and SJ Anderson. Aggregation and aggregate size effects on extractable iron and aluminm in two Hapludoxs. Soil Sci. Soc. Am. J. 1997; 61, 965-70.
CA Igwe. Erodibility in relation to water-dispersible clay for some soils of Eastern Nigeria. Land Degrad. Develop. 2005; 16, 87-96.
E Barberis, FA Marsan, V Boero and E Arduino. Aggregation of soil particles by iron oxides in various size fractions of soil horizons. J. Soil Sci. 1991; 42, 535-42.
CJ Bronick and R Lal. Soil structure and management: a review. Geoderma 2005; 124, 3-22.
JSC Mbagwu and U Schwertmann. Some factors affecting clay dispersion and aggregate stability in selected soil of Nigeria. Intern. Agrophysics 2006; 20, 23-30.
D Pinheiro-Dick and U Schwertmann. Microaggregates from oxisols and inceptisols: dispersion through selective dissolutions and physicochemical treatments. Geoderma 1996; 74, 49-63.
RC Johnes and G Uehara. Amorphous coatings on mineral surfaces. Soil Sci. Soc. Am. Proc. 1973; 37, 792-8.
JSC Mbagwu and P Bazzoffi. Effect of freezing and thawing on the stability of soil aggregates treated with organic wasters. Clod Regions Sci. Tech. 1989, DOI: 10.1016/0165-232x(89)90020-7.
RPC Morgan. Soil Erosion. In: Topics in Applied Geography. Longman Group, London, 1979, p. 113.
JD Oster, I Shainberg and JD Wood. Flocculation value and gel structure of sodium/calcium montmorillonite and illite suspensions. Soil Sci. Soc. Am. J. 1980; 44, 955-9.
RSN Green, AM Posner and JP Quirk. A Study of the Coagulation of Montmorillonite and Illite Suspensions by Calcium Chloride using the Electron Microscope. In: WW Emerson and RD Bond (eds.). Modification of Soil Structure. Wiley, New York, 1980, p. 35-40.
RH Stern, M Ben-Hur and I Shainberg. Clay mineralogy effect on rain infiltration, seal formation and soil losses. Soil Sci. 1991; 152, 455-62.
J Six, ET Elliott and K Paustian. Soil structure and soil organic matter: II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000; 64, 1042-9.
CA Igwe, FOR Akamigbo and JSC Mbagwu. Physical properties of soils of southeastern Nigeria and the role of some aggregating agents in their stability. Soil Sci. 1995; 160, 431-41.
GW Thomas. Exchangeable Cations. In: Page AL et al. (eds.). Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. 2nd ed. Madison, Wisconsin, United States, 1987, p. 159-65.
O Mehra and P Jackson. Iron oxide removal from soils and clays in a dithionite-citrate-bicarbonate system buffered with sodium. Clay. Clay Miner. 1960; 7, 317-27.
JA McKeague and JH Day. Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 1966; 46, 13-22.
JA McKeague. An evaluation of 0.1 M pyrophosphate and pyrophosphate-dithionite in comparison with oxalate as extractants of the accumulation product in podzols and some other soils. Can. J. Soil Sci. 1967; 47, 95-9.
K Norrish and JT Hutton. An accurate X-ray spectrographic method for the analysis of a wide range of geological samples. Geochim. Cosmochim. Acta 1969; 33, 431-53.
GW Gee and JW Bauder. Particle-sized analysis. In: Kulte A et al. (eds.). Method of Soil Analysis, Part 1: Physical and Mineralogical Methods. 2nd ed. Madison, Wisconsin, United States, 1986, p. 383-411.
ET Elliott. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986; 50, 627-33.
CO Márquez, VJ Garcia, CA Cambardella, RC Schultz and TM Isenhart. Aggregate-size stability distribution and soil stability. Soil Sci. Soc. Am. J. 2004; 68, 725-35.
RE Youker and JL McGuinness. A short method of obtaining mean weight diameter values of aggregate analyses of soils. Soil Sci. 1956; 83, 291-4.
HP Klug and LE Alexander. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials. John Wiley and Sons, New York, 1974, p. 99-116.
CA Igwe, M Zarei and K Stahr. Colloidal stability in some tropical soils of southeastern Nigeria as affected by iron and aluminum oxides. Catena 2009; 77, 232-7.
RJ MaCracken, RB Daniels and WE Fulcher. Undisturbed soils, landscape, and vegetation in a North Carolina piedmont virgin forest. Soil Sci. Soc. Am. J. 1989; 53, 1146-52.
P Trakoonyingcharoen, I Kheoruenromne, A Suddhiprakarn and RJ Gilkes. Properties of iron oxides in Thai red Oxisols and red Ultisols. Aust. J. Soil Res. 2006; 44, 63-70.
A Piccolo, G Pietramellara and JSC Mbagwu. Use of humic substances as soil conditioners to increase aggregate stability. Geoderma 1997; 75, 267-77.
P Trakoonyingcharoen, I Kheoruenromne, A Suddhiprakarn and RJ Gilkes. Properties of kaolins in red Oxisols and red Ultisols in Thailand. Appl. Clay Sci. 2006; 32, 25-39.
MN Arca and SB Weed. Soil aggregation and porosity in relation to contents of free iron oxide and clay. Soil Sci. 1996; 101, 164-70.
M Arias, MT Barral and F Diaz-Fierros. Effects of Organic Matter, Iron and Aluminium on Soil Structural Stability. In: Berthelm et al. (eds.). Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments. Kluwer Academic, Pienum Publishers, New York, 1999, p. 79-88.
S Imhoff, AP da Silva and A Dexter. Factors contributing to the tensile strength and friability of Oxisols. Soil Sci. Soc. Am. J. 2002; 66, 1656-61.
J Six, C Feller, K Denef, SM Ogle and MJC Sa. Soil organic matter, biota and aggregation in temperate and tropical soils-effects of two no-tillage. Agronomie 2002; 22, 755-75.
AP Edwards and JM Bremner. Microaggregates in soils. J. Soil Sci. 1967; 18, 65-73.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2012 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









