Automatic Screening of Lung Diseases by 3D Active Contour Method for Inhomogeneous Motion Estimation in CT Image Pairs
DOI:
https://doi.org/10.48048/wjst.2021.10573Keywords:
3D active contour model, lung disease analysis, inhomogeneous motion pattern, velocity vector map, hierarchical classificationAbstract
Lung diseases are now the third leading cause of death worldwide because of the many risk factors we are exposed to daily, such as air pollution, tobacco use, viruses (such as COVID-19), and bacteria. This work introduces a new approach of the 3D Active Contour Model (3D ACM) to estimate an inhomogeneous motion of lungs, which can be used to analyze lung disease patterns using a hierarchical predictive model. The biophysical model of lungs consists of End Expiratory (EE) and End Inspiratory (EI) models, generated by high-resolution computed tomography images (HRCT). A proposed technique uses the 3D ACM to estimate the velocity vector by using the corresponding points on the parametric surface model of the EE model to the EI model. The external energy from the EI models is the external force that pushes the 3D parametric surface to reach the boundary. The external forces, such as the balloon force and Gradient Vector Flow (GVF), were adjusted adaptively based on the which was calculated from the ratio of the maximum value of EI to EE on the Z axis. Next, the feature representation is studied and evaluated based on the lung structure, separated into five lobes. The stepwise regression, Support Vector Machine (SVM), and Artificial Neural Network (ANN) techniques are applied to classify the lung diseases into normal, obstructive lung, and restrictive lung diseases. In conclusion, the inhomogeneous motion pattern of lungs integrated with medical-based knowledge can be used to analyze lung diseases by differentiating normal and abnormal motion patterns and separating restrictive and obstructive lung diseases.
HIGHLIGHTS
- Inhomogeneous motion analysis from the expanding and shrinking lungs of HRCT pair
- Adaptive 3D Active Contour Model (ACM) for detecting the shape of the lung by balancing the balloon force with the stopping condition
- Lung lopes separation using oblique fissure and anatomical location
- Structure the velocity vector map of lung motion using bag of words of the magnitude
- Neural Network model for predicting obstructive and restrictive lung diseases
GRAPHICAL ABSTRACT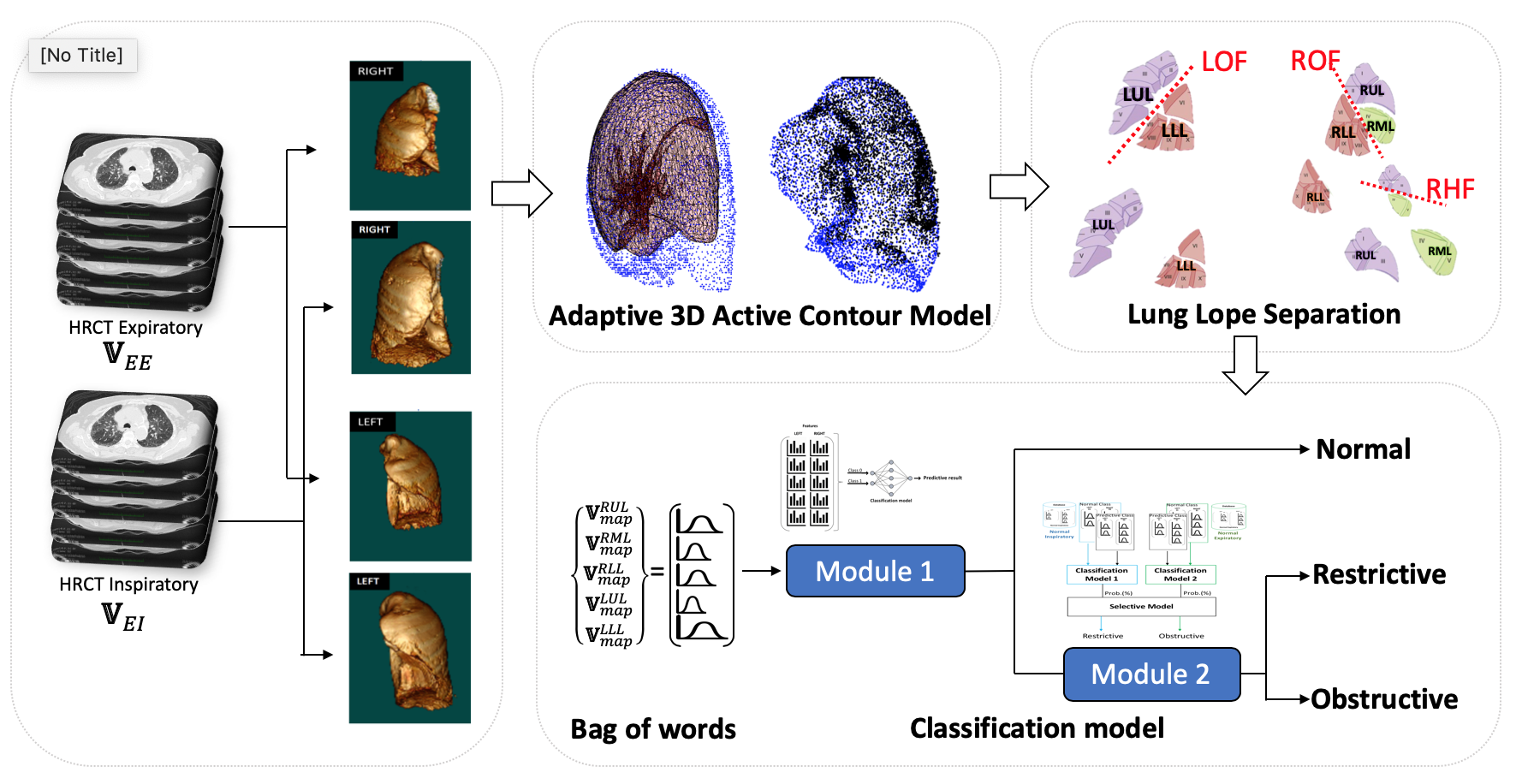
Downloads
Metrics
References
World Health Organization. The top 10 causes of death. World Health Organization. Available at: https://www.who.int, accessed June 2020.
SF Nemec, AA Bankier and RL Eisenberg. Upper lube-predominant diseases of the lung. Amer. J. Roentgenol. 2013; 200, W222-W237.
JD Backer. FRI and disease characterization: Idiopathic pulmonary fibrosis. Fluidda, 2018.
AJ Blaivas and W Strauss. Middle Lobe Syndrome in the Left Lower Lobe in Chronic Obstructive Pulmonary Disease. Prim. Care Respir. J. 2019; 18, 331-3.
SF Nemec, AA Bankier and RL Eisenberg. Lower lube: Predominant diseases of the lung. Amer. J. Roentgenol. 2013; 200, 712-28.
Y Qu, Y Cao, M Liao and Z Lu. Sagittal-lung CT measurements in the evaluation of asthma-COPD overlap syndrome: A distinctive phenotype from COPD alone. J. Italian Soc. Med. Radiol. 2017; 122, 487-97.
RA Hartley, BL Barker, C Newby, M Pakkal, S Baldi, R Kajekar, R Kay, M Laurencin, RP Marshall, AR Sousa, H Parmar, S Siddiqui, S Gupta and CE Brightling. Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: A single-center study. J. Allergy Clin. Immunol. 2016; 137, 5.
M Zehtabian, R Faghihi, MA Mosleh-Shirazi, AR Shakibafard, M Mohammadi and M Baradaran-Ghahfarokhi. A fast model for prediction of respiratory lung motion for image-guided radiotherapy: A feasibility study, Iran. J. Radiat. Res. 2012; 10, 73-81.
H Ladjal, N Skendraoui, M Giroux, Y Touileb, J Azencot, B Shariat, H Ladjal, M Beuve and P Giraud. Physiology and biomechanical model of patient specific lung motion based on 4D CT images. In: Proceedings of the 2015 Biomedical Engineering International Conference. Pattaya, Thailand, 2015, p. 1-5.
L Han, H Dong, JR McClelland, L Han, DJ Hawkes and DC Barratt. A hybrid patient-specific biomechanical model based image registration method for the motion estimation of lungs. Med. Image Anal. 2017; 29, 87-100.
CP Hersh, GR Washko and RSJ Estepar. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir. Res. 2013; 14, 42.
B Fuerst, T Mansi, F Carnis, M Salzle, J Zhang, J Declerck, T Boettger, J Bayouth, N Navab and A Kamen. Patient-specific biomedical model for the prediction of lung motion from 4-D CT images. IEEE Trans. Med. Imag. 2015; 34, 2.
Q Wei and Y Hu. A fybrid approach to segmentation of diseases lung lobes. IEEE J. Biomed. Health Inform. 2014; 18, 3.
S Ukil and JM Reinhardt. Anatomy-guided lung lobe segmentation in X-ray CT images. IEEE Trans. Med. Imag. 2009; 28, 2.
Z Zhang, X Ma and Y Yang. Bounds on the number of hidden neurons in three-layer binary neural networks. Neural Netw. 2003; 16, 995-1002.
D Hunter, H Yu, MS Pukish III, J Kolbusz and BM Wilamowski. Selection of proper neural network sizes and architectures: A comparative study. IEEE Trans. Ind. Inform. 2012; 8, 228-40.
K Shibata and Y Ikeda. Effect of number of hidden neurons on learning in large-scale layered neural networks. In: Proceedings of the ICROS-SICE International Joint Conference 2009. Fukuoka, Japan, 2009, p. 5008-13.
PP Walker, P Mitchell, F Diamantea, CJ Warburton and L Davies. Effect of primary-care spirometer on the diagnosis and management COPD. Eur. Respir. J. 2006; 28, 945-52.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.













